Lastly, owning the wrong freeze drying equipment to your lyophilization process can also be a giant mistake. Do you want a stopper sample less than your vacuum? Then a stopping chamber is required. Have you been freeze-drying in flasks? Then you'll want to have a drying chamber with ports.
Eutectic Stage or Eutectic Temperature Is The purpose at which the product or service only exists during the good stage, symbolizing the least melting temperature. Not all products Possess a eutectic point or there may be multiple eutectic factors.
A modify in vial involves only the willpower from the Kv for that vial and incorporating the information in the present graph When the fill volume won't dramatically change as Rp is often a purpose of fill volume.
There exists a 20% amplified possibility of atrial fibrillation, among the individuals who described ingesting two liters or more each week of artificially sweetened…
Merchandise load or batch dimensions influences the process performance, especially the key drying time and warmth transfer coefficient, and regulatory organizations assume revalidation on the process when the batch dimensions is altered from throughout the validated selection. By way of example, partial load drying process have been done on LabLyo1 with one hundred%, ten%, 5%, and a couple of% loads, plus the related heat transfer coefficient, Kv, adjustments throughout load dimensions were analyzed working with very first principles heat transfer design outlined in before discussions.
Rp and Kv are combined with the important product temperature for your merchandise and the products ability curve to create a style Area graph. The calculations are entered into an Excel® macro (or equal program) to unravel the model equations and compute the products temperature at distinct combos of shelf temperature and chamber stress. The calculations can be performed iteratively to build the look Place graph. Normally, various strategies may be used to accomplish the calculations for any style and design Place so long as These are made dependant on the most crucial equations for Rp and Kv presented previously mentioned (seventeen).
Lyophilization guarantees vaccine stability and potency, which can read more help with around the world immunization initiatives.
When plant-primarily based foods are the most popular freeze-dried products, a wide array of foods can be preserved working with this process.
Maintain moments and cooling ramp charges could possibly be critical in reducing the variability of ice nucleation and crystal expansion. Some reports propose that some molecules could possibly be sensitive to prolonged residence occasions in the freeze focus earlier mentioned the glass transition (Tg’) and may adversely effect stability.
This Web-site works by using cookies to enhance your experience while you navigate via the website. Out of these, the cookies that happen to be classified as vital are saved on the browser as They're essential for the Operating of basic functionalities of the web site.
Depending upon the business, these runs can be often called engineering, development, or demonstration operates, but in all scenarios, the lyophilization process, in addition to other device functions inside the formulation, filling, and inspection, is staying examined to detect any surprising changes That may take place in the transfer from smaller-scale runs or in tech transfer to a completely new website.
Higher money financial investment: Big-scale lyophilization for sterile products calls for multi-million dollar investments into equipment and facility maintenance (learn more about sterile manufacturing and aseptic processing here). Because of this, both small and huge pharmaceutical providers will often transfer their lyophilization processes to contract improvement and producing businesses (CDMOs) for scientific and business producing.
The Lyo-Works Operating Procedure tends to make freeze drying easy. The big, total shade touchscreen Screen provides very click here clear, intuitive interactions. Learn how to use these characteristics that assist you get the lyophilization final results you would like when.
Managing the temperature at which ice nucleates can considerably lessen the variability amongst the vials on a shelf and concerning shelves, both equally at tiny scale and at full scale. Cutting down the variability can make certain all product in all vials dry at an identical level and should exhibit identical top quality characteristics such as appearance residual moisture and reconstitution time. This will have an additional benefit of considerably minimizing Most important drying time. The possibilities for lowering variability and lyophilization processing time have enhanced the desire of pharmaceutical companies in CIN.
 Luke Perry Then & Now!
Luke Perry Then & Now!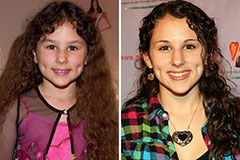 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Raquel Welch Then & Now!
Raquel Welch Then & Now! Richard Thomas Then & Now!
Richard Thomas Then & Now! Meadow Walker Then & Now!
Meadow Walker Then & Now!