Any deviations or tendencies which could possibly effect product or service quality has to be identified and resolved promptly.
Validation performs an important position inside the drug improvement and manufacturing lifecycle. All units, devices, processes, and procedures that have GxP influence call for some type of validation.
A: The Nationwide Institute of Most cancers’s validation summary report definition is: A summary of all planned pursuits, their accomplishment or failure, and any deviations in the expected outcomes or designs encountered. A satisfactory resolution really should be presented to elucidate and take care of any deviations encountered.
This is very essential With regards to measuring critical good quality characteristics of drug solutions, as these characteristics instantly influence affected person safety and merchandise good quality.
Process validation employs aim proof to ascertain the process is repeatable and, importantly, to determine the way it is repeatable. The process can help test variability to make certain that various inputs will carry on to yield constant product top quality.
Process validation will help companies maintain transparency with regulatory bodies and comply with present-day legal guidelines.
The innovation-driven articles management platform to supply and distribute premium quality electronic articles
IQ entails verifying that the tools is set up the right way and according to the maker's specs. This ensures that the machines is in the proper affliction to perform its meant functions.
How did you solicit and include suggestions out of your friends, supervisors, or clientele? How did you make sure your report fulfills the moral and Skilled requirements within your area and Group? By examining and revising your report, you are going to make sure your report is of top of the range and effects.
It’s not on a professional subject It contains inaccuracies It's offensive language It's got hazardous advice It is made up of stereotypes or bias It’s redundant and unclear Translation quality is inadequate It’s not suitable in my nation, area or society If you think one thing in the following paragraphs goes against our Skilled Local community Procedures, be sure to allow us to know.
Make a report outline for your approval report that you would wish to create. By doing this, you must also analyze the kind of data you would like to generate. Mature your choices Using these report outline and just spot your other facts in the report. You may as well check here Look at report the report templates.
Top quality teams need to know which characteristics to monitor to ensure the production process runs efficiently. That may be why many corporations turn to data analytics to pinpoint the parameters that impact creation probably the most.
Process validation might be labeled In accordance with when groups carry out checks and what their goal is. The types incorporate:
Validation of analytical strategies is essential for compliance and ensuring the efficacy check here of pharmaceutical items. Regulatory businesses including the FDA need companies to display that their analytical strategies are scientifically seem and able of producing trustworthy benefits.
 Mr. T Then & Now!
Mr. T Then & Now! Alfonso Ribeiro Then & Now!
Alfonso Ribeiro Then & Now!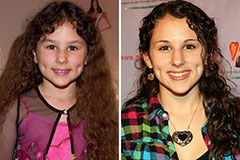 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Elisabeth Shue Then & Now!
Elisabeth Shue Then & Now! Brooke Shields Then & Now!
Brooke Shields Then & Now!